|
Article Information
|
Authors:
Gordon J. Gallivan1
Andrea Spickett2
Heloise Heyne2
Arthur M. Spickett2
Ivan G. Horak3,4
Affiliations:
1187 Cluny Street, Ottawa, Canada
2Parasites, Vectors & Vector-borne Diseases Programme, Onderstepoort Veterinary Institute, South Africa
3Department of Veterinary Tropical Diseases, University of Pretoria, South Africa
4Department of Zoology and Entomology, University of the Free State, South Africa
Correspondence to:
Ivan Horak
Email:
ivan.horak@up.ac.za
Postal address:
Private bag X04, Onderstepoort 0110,
South Africa
Dates:
Received: 09 Sept. 2010
Accepted: 02 Feb. 2011
Published: 19 Apr. 2011
How to cite this article:
Gallivan, G.J., Spickett, A., Heyne, H., Spickett, A.M. & Horak, I.G., 2011, ‘The dynamics of
questing ticks collected for 164 consecutive months off the vegetation of two landscape zones in the
Kruger National Park (1988–2002). Part III. The less commonly collected species’,
Onderstepoort Journal of Veterinary Research 78(1): Art. #41, 9 pages.
doi:10.4102/ojvr.v78i1.41
Copyright Notice:
© 2011. The Authors. Licensee: OpenJournals Publishing. This work
is licensed under the Creative Commons Attribution License.
ISSN:
0030-2465 (print)
ISSN:
2219-0635 (online)
|
|
|
|
The dynamics of questing ticks collected for 164 consecutive months off the vegetation
of two landscape zones in the Kruger National Park (1988–2002). Part III. The
less commonly collected species
|
|
In This Original Research...
|
Open Access
|
• Abstract
• Introduction
• Materials and methods
• Results and discussion
• Amblyomma marmoreum
• Dermacentor rhinocerinus
• Haemaphysalis elliptica
• Hyalomma truncatum
• Rhipicephalus evertsi evertsi
• Rhipicephalus simus
• Rhipicephalus turanicus
• Conclusion
• Acknowledgements
• References
|
|
Despite many studies regarding tick ecology, limited information on long-term changes in tick
populations exist. This study assessed the long-term population dynamics of the less frequently
collected questing ixodid ticks in the Kruger National Park (KNP). From 1988 to 2002, monthly
dragging of the vegetation was performed in three habitats (grassland, woodland and gully) at
two sites in the KNP (Nhlowa Road, Landscape Zone 17, and Skukuza, Landscape Zone 4).
Amblyomma marmoreum and Rhipicephalus evertsi evertsi were collected
as larvae most commonly. Most A. marmoreum larvae were collected
at Skukuza and numbers peaked from March to July. More R. evertsi evertsi
larvae were collected at Nhlowa Road and numbers peaked in summer and in winter, while at
Skukuza there was a single peak in spring. Haemaphysalis elliptica, Rhipicephalus
simus and Rhipicephalus turanicus were collected as adults most commonly. More
Ha. elliptica and R. turanicus were collected at Nhlowa Road than at Skukuza,
while R. simus numbers from the two sites were approximately equal.
Ha. elliptica were collected most often between February and June, and R. simus
and R. turanicus during February and March. All three species were collected more
frequently in gullies than in grassland or woodland. Their numbers increased in 1994/1995 following
an eruption of rodents, the preferred hosts of the immature stages. The different host-seeking
strategies of ticks largely determine the development stage at which they are likely to be
collected during vegetation dragging and reflect a complex interaction between ticks, their
hosts and the environment.
Many studies have examined the association between ticks, their hosts and the environment, but most have
been of relatively short duration (typically 1–2 years) and there is little information on longer-term
changes in tick populations. In earlier publications (Horak, Gallivan & Spickett 2011; Spickett,
Gallivan & Horak 2011) we described changes in the numbers of questing ticks and the population
dynamics of the four major tick species, Amblyomma hebraeum, Rhipicephalus appendiculatus,
Rhipicephalus decoloratus and Rhipicephalus zambeziensis. The questing ticks were collected
monthly by dragging flannel strips over the vegetation at two sites in the Kruger National Park (KNP) over
a period of 164 months. In the present publication we describe changes in the numbers of the less commonly
collected species, namely Amblyomma marmoreum, Dermacentor rhinocerinus, Haemaphysalis
elliptica, Hyalomma truncatum, Rhipicephalus evertsi evertsi, Rhipicephalus simus
and Rhipicephalus turanicus.
The KNP is a large nature reserve of nearly 2 million ha in north-eastern South Africa. Five vegetation types
(Acocks 1988) and 35 Landscape Zones (Gertenbach 1983) have been identified in the reserve. The study sites
at Skukuza and Nhlowa Road and the associated climate, methods of tick collection and statistical analyses
have been described in detail by Horak et al. (2011). The climate in the southern KNP is described as
tropical with summer rainfall. Annual rainfall is measured from June to May; consequently, we have chosen
June to represent the commencement of each year.
Briefly, questing ticks were collected monthly by drag-sampling the vegetation with flannel strips
(Spickett et al. 1992) at two sites in the KNP, namely Nhlowa Road and Skukuza. The Nhlowa Road
site was in Landscape Zone 17, which has been described as a Sclerocarya caffra – Acacia
nigrescens savanna, and the Skukuza site in Landscape Zone 4 (Thickets of the Sabie and
Crocodile Rivers). Three drags, approximately 250 m long and 1 m wide, were made at a normal walking
speed in each of three visually selected habitats (grassland, woodland and gully) at each site. In
addition to the flannel strips, the operator responsible for dragging the strips also wore flannel
leggings. Because of the limited number of gullies at each site, the same gullies were usually sampled
every month, whereas the sampling locations in grassland and woodland usually varied from month to month.
After each drag the ticks on the flannel strips and the leggings were removed using sharp-pointed forceps
and stored in 70% ethyl alcohol in internally labelled, plastic-stoppered glass vials for later identification
and quantification.
Sampling commenced in August 1988 and continued in every habitat at the two localities until March 2002,
except after heavy rainfall, during a bush fire or upon sighting elephants (Loxodonta africana),
African buffaloes (Syncerus caffer) or lions (Panthera leo) in the vicinity.
The identification of the immature stages of ticks, particularly of the lesser-known species, can be a
difficult and time-consuming exercise. The larvae and nymphs of A. marmoreum have been described
by Theiler and Salisbury (1959), those of D. rhinocerinus by Keirans (1993), of Ha. elliptica
by Apanaskevich, Horak and Camicas (2007), of Hy. truncatum by Apanaskevich and Horak (2008), and of
the various Rhipicephalus species by Walker, Keirans and Horak (2000).
Annual rainfall (June–May) at the Skukuza rest camp averaged 609 mm over the period of the study,
with a range of 275 mm – 1122 mm (Figure 1a). Average annual rainfall at the Lower Sabie rest camp,
the closest climate station to the Nhlowa Road site, was similar to that at Skukuza. Mean monthly maximum
temperatures fluctuated around 33 °C in mid-summer, while minimums rarely fell below 5 °C
in winter (Figure 1b).
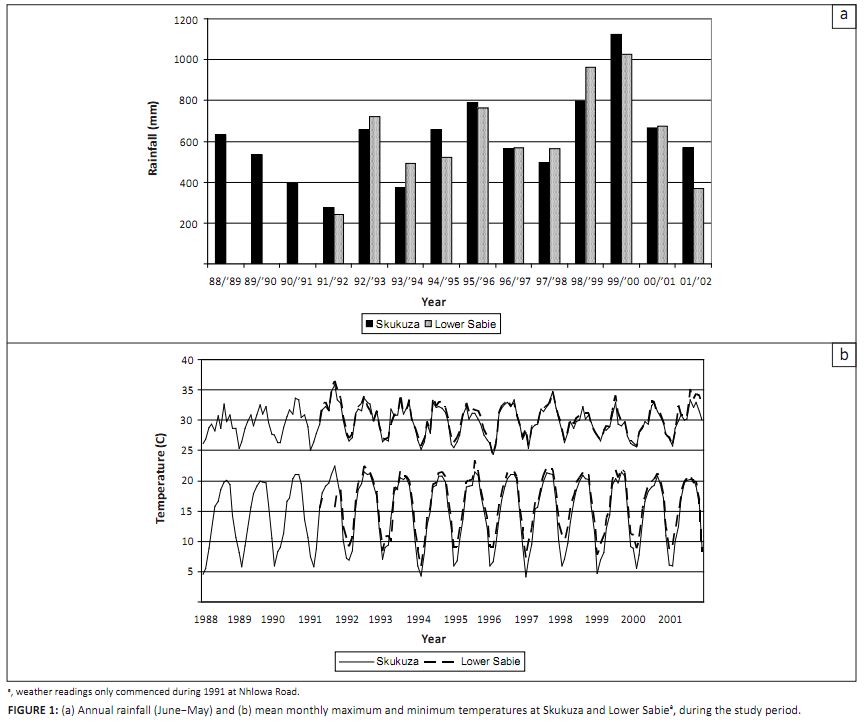 |
FIGURE 1: (a) Annual rainfall (June-May) and (b) mean monthly maximum and minimum temperatures at Skukuza and Lower Sabie , during the study period.
|
|
The numbers of all species collected from the vegetation are summarised in Table 1. Four taxa could be
identified only to a generic level, including 188 Amblyomma sp. larvae, 104 Haemaphysalis
sp. larvae, a single Ixodes sp. nymph and a single Rhipicephalus pravus group larva.
Counts of specimens of these four taxa and those of the four major tick species, namely A. hebraeum,
R. appendiculatus, R. decoloratus and R. zambeziensis are listed collectively as ‘
other species’ in the table. Population dynamics of the four major tick species have been discussed
in earlier publications (Horak et al. 2011; Spickett et al. 2011). Except for Ha.
elliptica, R. simus and R. turanicus, of which adults were the most commonly collected
stage, larvae were the most commonly collected stage of all these tick species.
|
TABLE 1: Numbers of the less common tick species collected by dragging at the Skukuza and Nhlowa Road sites in the Kruger National Park (August 1988–March 2002).
|
Amblyomma marmoreum
Leopard tortoises (Geochelone pardalis), the largest tortoise species in South Africa (Branch 1998),
are the preferred hosts of the adults of A. marmoreum and hence it is colloquially known as the South
African tortoise tick (Horak et al. 2006a). Adult ticks are rarely found on hosts other than reptiles,
but the larvae infest a wide range of hosts, including carnivores, herbivores, hares, birds and reptiles
(Horak et al. 2006a).
Amblyomma marmoreum larvae were collected more frequently at Skukuza than at Nhlowa Road (p < 0.001)
and accounted for 98.8% of the A. marmoreum specimens collected (Table 1). The riverine thickets at
Skukuza probably afforded greater protection for younger tortoises against predators and this may explain why larvae
were collected more frequently at Skukuza than at Nhlowa Road. The collections were seasonal (p < 0.001),
with most larvae collected between March and July. There was a definite peak in May at Skukuza but not at Nhlowa Road
(Figure 2a). This corresponds to the seasonality of A. marmoreum larvae on greater kudus (Tragelaphus
strepsiceros), impalas (Aepyceros melampus), scrub hares (Lepus saxatilis) and helmeted
guineafowls (Numida meleagris) examined in the KNP (Horak et al. 1991; Horak et al. 1992;
Horak et al. 1993; Horak et al. 1995; Horak et al. 2003), and countrywide on leopard
tortoises (Horak et al. 2006a).
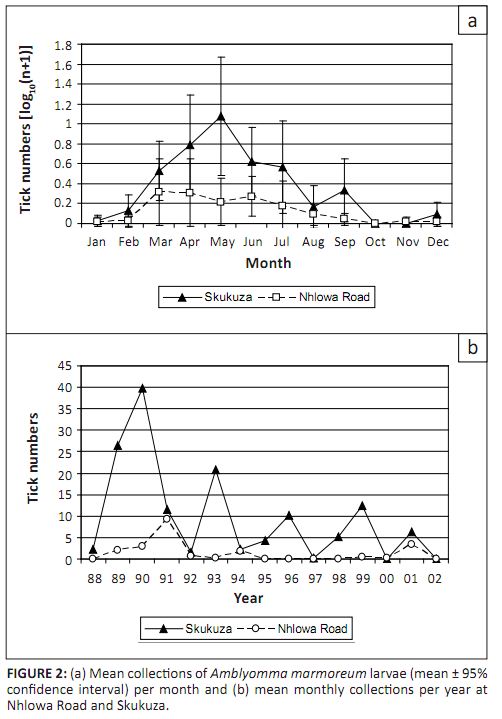 |
FIGURE 2: (a) Mean collections of Amblyomma marmoreum larvae (mean ± 95%
confidence interval) per month and (b) mean monthly collections per year at
Nhlowa Road and Skukuza.
|
|
Although there was considerable year-to-year variability, the numbers of A. marmoreum larvae collected
each year appeared to decline from 1990 to 2001 (Figure 2b). The size of the collections was not significantly
correlated with annual rainfall or rainfall over the activity period (February–September). The numbers
did not differ significantly among habitats (p = 0.20), but there was a trend towards more frequent collections
in the gullies than in grassland and woodland (Table 2). The slightly more frequent collections of A. marmoreum
larvae in gullies could imply that tortoises prefer to browse on the more succulent vegetation usually found there.
|
TABLE 2: Mean monthly tick collections [log10 (n + 1)] by habitat.
|
Dermacentor rhinocerinus
Adult D. rhinocerinus are found almost exclusively on black and white rhinoceroses (Diceros
bicornis and Ceratotherium simum) (Keirans 1993), of which nearly all in the southern KNP are
infested and on which the tick may occur in large numbers (Knapp et al. 1997). Adult ticks quest
for their preferred hosts at a height of 1 m or more on thick-stemmed, tall grass species (Horak, personal
observations 2000). They are seldom picked up on flannel strips and only two adults were collected (Table 1).
Because adult ticks have a very strong affinity to rhinoceroses they have not been collected from any of
the numerous host species we examined in the KNP. It is this affinity that probably makes the operator
dragging the flannel strips and the strips themselves unattractive to the ticks.
The rodent hosts of the immature stages have been detected only recently (Horak & Cohen 2001). In
Mthethomusha Nature Reserve (adjacent to the KNP and where there are also white rhinoceroses) three
rodent species, namely bushveld gerbils (Tatera leucogaster), red veld rats (Aethomys chrysophilus)
and Natal multimammate mice (Mastomys natalensis) were found infested with the immature stages of D.
rhinocerinus (Horak & Cohen 2001). The small size of these animals implies that the immature stages of
D. rhinocerinus quest for hosts from the soil surface rather than the vegetation, and only seven larvae
were collected from the flannel strips (Table 1). No bushveld gerbils or red veld rats examined in the KNP were
infested (Braack et al. 1996).
Haemaphysalis elliptica
Haemaphysalis elliptica adults were more commonly collected at Nhlowa Road than at Skukuza (p = 0.001).
Landscape Zone 17 is at the centre of lion activity in the KNP (Gertenbach 1983), which may account for the greater
number of adult ticks in this area. There was a significant difference between the numbers of adults collected over
years (p < 0.001). Some were collected from 1988 to 1990, but few were collected from 1991 to 1993.
The numbers then increased, with a marked peak in 2000 (Figure 3a). The patterns were similar at both sites, but there
was a significant year-by-site interaction (p < 0.001), because more were collected at Skukuza in 1988 and
1989 while more were collected at Nhlowa Road in the other years. The collections of adult ticks were correlated with
annual rainfall of the preceding year at both sites (r > 0.67; p < 0.01). A significant correlation
with rainfall 2 years before was also observed at Skukuza (p = 0.64; p < 0.05), and a positive but
non-significant correlation with rainfall observed at Nhlowa road (p = 0.48; p < 0.2). The dramatic
increase in numbers in 1999 and 2000 followed 2 years of above-average rainfall in 1998 and 1999. Following a rodent
explosion in 1993 (Horak, Spickett & Braack 2000b), after above-average annual rainfall in 1992, numbers peaked
slightly at Nhlowa Road in 1994 and 1995. The increase in numbers of Ha. elliptica adults around the turn of
the century was probably also associated with an increase in rodent populations during a period of above-average rainfall.
According to the time series assessment, the seasonality of Ha. elliptica adults was not statistically significant,
probably because of the small numbers collected in most years. However, an ANOVA showed a significant difference between
months (p < 0.001), with the largest numbers collected in late summer and autumn (February–June) and the
smallest numbers in spring (September–November) (Figure 3b). A similar pattern was observed at both sites. There are
no data regarding the seasonality of adult Ha. elliptica on carnivores in the KNP, but in north-eastern KwaZulu-Natal,
south-east of the KNP, adult ticks on dogs in rural communities were found mostly from January to March or April (Horak,
Emslie & Spickett 2001).
Haemaphysalis elliptica larvae were more commonly collected at Skukuza than at Nhlowa Road. Most larvae were collected
in 1989 and 1990, and only sporadically thereafter. The largest numbers were collected in September and November, and only
two of the 60 were collected between January and April. Nymphs were collected sporadically in the early 1990s and again
towards the turn of the century. Eight of the 10 nymphs were collected between July and October. The September–November
and July–October peaks for collections of Ha. elliptica larvae and nymphs, respectively, correspond to the
seasonal peaks in Ha. elliptica/spinulosa larval and nymphal infestations on red veld rats in the southern KNP (Braack
et al. 1996).
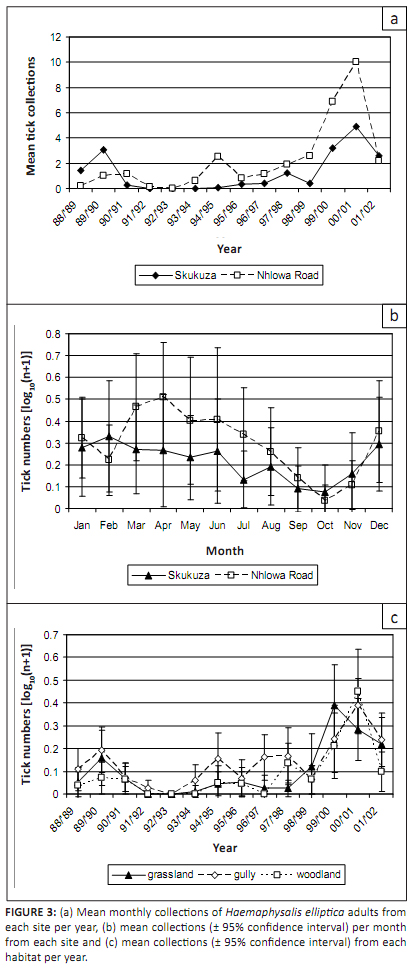 |
FIGURE 3: (a) Mean monthly collections of Haemaphysalis elliptica adults from
each site per year, (b) mean collections (± 95% confidence interval) per month
from each site and (c) mean collections (± 95% confidence interval) from each
habitat per year.
|
|
As shown in Table 2, the largest numbers of Ha. elliptica adults were collected in the gullies, while fewest were collected
in woodland (p < 0.01). This pattern was consistent over months and at both sites. However, there was a significant
difference between years (p = 0.02), with the largest numbers collected in the gullies until the late 1990s Figure 3c)
followed by more frequent collections in grassland and woodland. Eight of the ten nymphs were collected in the gullies.
Tall grasslands appear to support a higher diversity of small mammal populations (Monadjem 1997), but many of the rodent hosts
of the immature stages of Ha. elliptica prefer sheltered, rocky areas (Skinner & Smithers 1990). During periods of
below-average rainfall, when grass swards were shorter and ground cover was reduced, gullies were probably the preferred
habitat of many of the rodent hosts. Around the turn of the century the majority of Ha. elliptica adults were
collected in grassland and woodland. Denser ground cover and taller grass swards following a period of higher rainfall
would have provided a more suitable habitat for many rodent species, and an increase in rodent populations would have
led to an expansion of habitat use. Thus, the population dynamics and habitat distribution of questing Ha. elliptica
are probably determined by the effects of rainfall on the populations of rodent hosts of the immature stages, rather than
the carnivore hosts of the adults.
Hyalomma truncatum
Although only 24 larvae and five adult Hy. truncatum were collected from the vegetation (Table 1),
these low numbers should not be regarded as a reflection of actual abundance of this tick in the KNP. During
the first 6 years of this survey, five scrub hares were examined monthly around Skukuza, and the 360
examinations during this time yielded a total of 36 647 larvae and 9896 nymphs of this two-host tick
(Horak et al. 1993; Horak et al. 1995). Adult Hy. truncatum have been collected from
the large browsers and grazers in the KNP, including giraffes (Giraffa camelopardalis), Burchell’s
zebras (Equus burchellii), elands, (Taurotragus oryx), white rhinoceroses and kudus, as well as
warthogs (Phacochoerus africanus) (Horak, De Vos & De Klerk 1984; Horak et al. 1988; Horak
et al. 1992; Horak, Golezardy & Uys 2007; Knapp et al. 1997). The discrepancy between the
numbers collected by dragging and the numbers on scrub hares strongly suggests that Hy. truncatum larvae
quest for hosts from the soil surface. The adults definitely quest for hosts from the soil surface and can readily
be seen scuttling along the ground towards any large potential host.
Rhipicephalus evertsi evertsi
Rhipicephalus evertsi evertsi is a two-host tick that infests a wide range of host species (Walker et al. 2000).
In the KNP, Burchell’s zebras and giraffes appear to be preferred hosts for all stages (Horak et al. 1984; Horak
et al. 2007), while impalas and scrub hares are important hosts for the immature stages (Horak et al. 1993; Horak
et al. 1995; Horak et al. 2003). Larvae were most commonly collected during dragging (Table 1) and, theoretically,
only larvae and adults should quest for hosts. The single nymph that was collected had probably become dislodged just before or
after moulting and was questing for a host. Very few adults were collected, probably because they quest for hosts from the soil
surface or, like the adults of many species, they do not attach to flannel strips.
In previous drag-sampling studies in Landscape Zones 4 and 17 there was no evidence of seasonality in the numbers of questing
R. evertsi evertsi larvae collected (Spickett et al. 1992; Horak et al. 2006b). The present study, however,
showed significant periodicity at both sites (p < 0.001). At Nhlowa Road there were two annual peaks (November–
February and June–July), whereas at Skukuza, there was only a single peak (November–December) with a nadir between
May and June (Figure 4a). Based on the development times seen in the laboratory, R. evertsi evertsi can complete more
than one life cycle annually at the prevailing temperatures in the KNP (Horak et al. 2003). At Nhlowa Road the summer
and winter peaks correspond to those observed on Burchell’s zebras (Horak et al. 1984), suggesting that the seasonal
pattern was related to the pattern of zebra migration. At Skukuza, the late spring peak and late autumn nadir differed not only
from the pattern observed in other drag-sampling studies but also from that observed on impalas and scrub hares in this landscape
zone (Horak et al. 1993; Horak et al. 2003). The reasons for the differences between the seasonal patterns observed
in the present study and previous studies are unknown. They may be related to seasonal differences in habitat use by ungulates
within Landscape Zone 4, or they may be because of the relatively short duration of the sampling periods (1–4 years) in
the earlier studies. In the present study there was considerable variability among years and the general seasonal pattern was
seen only over the longer time period.
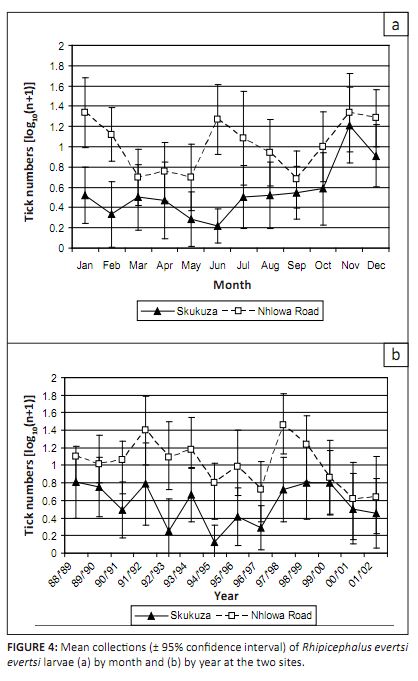 |
FIGURE 4: Mean collections (± 95% confidence interval) of Rhipicephalus evertsi
evertsi larvae (a) by month and (b) by year at the two sites.
|
|
The numbers of R. evertsi evertsi larvae collected differed significantly among years (p < 0.001) and
there was a significant year-by-site interaction (p = 0.028), with proportionally greater changes at Skukuza than
at Nhlowa Road. However, the trends were similar at both sites, with peaks in the early 1990s, a decline through the mid-1990s,
and subsequent increases in the late 1990s. Numbers declined in 2000 (Figure 4b). The numbers were not significantly correlated
with seasonal rainfall at either site. The apparent differences in the annual trends at the two sites may be related to
differences in the host communities. The populations of the larger ungulates at Nhlowa Road, such as zebras and giraffes,
appear to be less sensitive to changes in rainfall (Ogutu & Owen-Smith 2005) than the populations of smaller ungulates,
such as impalas, at Skukuza.
Larvae were more commonly collected at Nhlowa Road than at Skukuza (p < 0.001) and there was a significant
habitat-by-site interaction (p = 0.002). At Nhlowa Road, larvae were least frequently collected in the gullies
and most commonly in the grassland (Table 2), probably because of the large numbers of zebras and other grazers at this
locality. At Skukuza, R. evertsi evertsi larvae were most commonly collected in the woodland, probably
because of the presence of large numbers of impalas and other browsers, but the difference among habitats was not
statistically significant (p > 0.1). The differences among habitats were not significant among months or years
(p > 0.4).
Rhipicephalus simus
Adults were the most commonly collected stage of R. simus. The preferred hosts of adult R. simus are
large ruminants, such as African buffaloes, and monogastric animals, such as large carnivores, warthogs, zebras and
rhinoceroses (Walker et al. 2000). Large numbers of R. simus adults have been collected from lions,
leopards, warthogs, Burchell’s zebras and white rhinoceroses in the KNP (Horak et al. 1984; Horak et al.
1988; Horak et al. 2000a; Knapp et al. 1997).
The collections of R. simus were seasonal, with a peak between February and March and a nadir between August
and September (Figure 5a). The numbers did not differ significantly between the two sites (p = 0.2) and the
seasonal patterns were similar. The peak in adult numbers corresponds with that on Burchell’s zebras and warthogs
in the KNP (Horak et al. 1984; Horak et al. 1988) and on warthogs in Swaziland in a Sclerocarya
caffra – Acacia nigrescens savanna (Gallivan & Surgeoner 1995).
 |
FIGURE 5: Mean collections (± 95% confidence interval) of Rhipicephalus simus
adults (a) by month and (b) by year at Nhlowa Road and Skukuza.
|
|
There was a significant difference between the numbers of R. simus adults collected over the years (p < 0.001).
Numbers declined from 1988 to 1991, then increased again in 1994, followed by a peak in 2000 (Figure 5b). The trends differed
significantly between the two sites (p < 0.001). At Skukuza, numbers tended to be higher between 1988 and 1989 than
from 1997 to 2000. However, at Nhlowa Road the numbers of R. simus adults were higher from 1993 to 1995, with a marked
peak in 1994.
The numbers of adult R. simus collected at Skukuza were significantly correlated with annual rainfall during the
2 previous years (p < 0.05). At Nhlowa Road, the numbers were significantly correlated with rainfall 2 years
before (p = 0.05), but not with that of the preceding year. The decline in numbers in the early 1990s corresponded
to a period of decreasing annual rainfall and to a marked decline in the warthog population in the KNP (Ogutu & Owen-Smith 2005).
The decline in annual rainfall probably also caused a decline in the populations of rodents, which are the preferred hosts of
the immature stages of this tick (Braack et al. 1996; Horak et al. 2005; Norval & Mason 1981; Petney et al. 2004).
Following a period of above-average rainfall in 1992, there was an eruption of rodent populations in 1993 (Horak et al.
2000b) that may have accounted for the large number of adult R. simus collected at Nhlowa Road during 1994. As with the
adults of Ha. elliptica, the increase in the numbers of R. simus adults around the turn of the century was
probably associated with an increase in rodent populations during a period of above-average rainfall.
As shown in Table 2, adult R. simus were most commonly collected in the gullies and least frequently in the grassland
(p < 0.001). There was a significant difference in the habitat distribution between the two sites (p = 0.03).
At Skukuza, 48.3% of the ticks were collected in the gullies and 16.4% in grassland, whereas at Nhlowa Road, 69.2% were
collected in the gullies and only 7.6% in grassland. Habitat distribution differed significantly among months (p = 0.025),
with the most pronounced difference during February and March, the period of peak activity. There was also a marginally significant
difference in habitat distribution among years (p = 0.1), with highest numbers in the gullies; however, in 2000 the numbers
were marginally higher in woodland. As in the case of Ha. elliptica, the habitat distribution probably corresponds to the
pattern of habitat use by the hosts of the immature stages.
Rhipicephalus simus larvae were more frequently collected at Skukuza than at Nhlowa Road. Almost half (45.8%) were
collected in 1989, while the second largest collection (14.6%) was made in 2001. Other collections were sporadic. At
Skukuza, most R. simus larvae were collected from January to June, but at Nhlowa Road most were collected from
June to November. At Skukuza, more larvae were collected in the gullies than in grassland, whereas at Nhlowa Road, most
larvae were collected in woodland.
Rhipicephalus turanicus
Adult R. turanicus have been collected from lions, leopards, cheetahs, African wild dogs, feral cats and
scrub hares in the KNP, but none of the animals were heavily infested (Horak et al. 1993; Horak et al.
1995; Horak et al. 2000a). No immature stages have been collected from animal hosts within the KNP or adjacent
nature reserves. Scrub hares examined on farms around Hluhluwe in north-eastern KwaZulu-Natal were infested with all
stages of R. turanicus (Horak et al. 1995) and crested francolins (Francolinus sephaena) on a
farm in Limpopo were infested with larvae (Uys & Horak 2005). Adult ticks were also common on dogs in Maputo Province,
Mozambique, adjacent to the KNP (Neves, Afonso & Horak 2004).
Rhipicephalus turanicus was most commonly collected at Nhlowa Road (97.8%) and adults were the most commonly
collected stage (Table 1). As mentioned before, Landscape Zone 17 is at the centre of lion distribution within the
KNP (Gertenbach 1983) and the herds of large grazers would support populations of other carnivores. Collections were
seasonal (p < 0.001), with a peak between February and March (Figure 6a). There were significant differences
between the numbers collected among years (p = 0.002), with peaks in 1991, 1994, 1995, 1999, 2001 and 2002
(Figure 6b). The numbers of R. turanicus adults were not correlated with rainfall and, as shown in Table 2,
ticks of this stage were collected in the gullies more often than in woodland (p < 0.001). This pattern was
consistent among years and months.
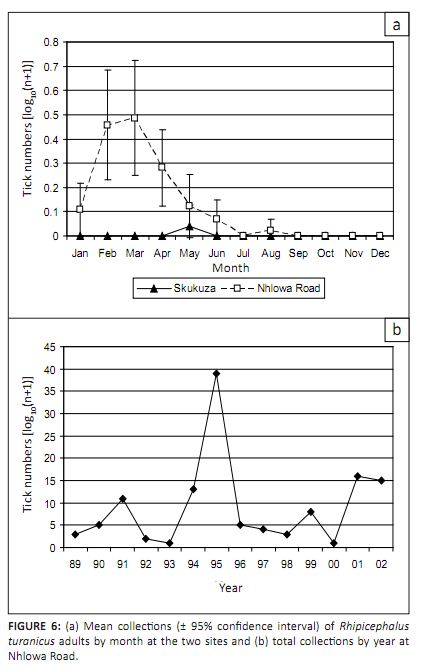 |
FIGURE 6: (a) Mean collections (± 95% confidence interval) of Rhipicephalus
turanicus adults by month at the two sites and (b) total collections by year at
Nhlowa Road.
|
|
There was a strong correlation (r = 0.7; p = 0.009) between the numbers of adult R. simus and
adult R. turanicus collected annually at Nhlowa Road. The year-to-year patterns of the two species were similar
and both species were most commonly collected in the gullies. However, woodland yielded the fewest R. turanicus
adults, whereas the grassland yielded the fewest R. simus adults. The distribution of adult R. turanicus
among habitats and year-to-year collection patterns were also similar to those of adult Ha. elliptica at the
Nhlowa Road site.
In order to determine the population dynamics of free-living ixodid ticks, questing ticks were collected monthly for
164 months from the vegetation at two localities in the KNP. Of the collected species, 14 could be identified to specific
level. Larvae of A. marmoreum and R. evertsi evertsi, and adults of Ha. elliptica, R. simus
and R. turanicus were collected in sufficient numbers to conclude that they quest for hosts from the vegetation.
The host-seeking strategies of their remaining developmental stages and those of the tick species collected only in small
numbers still need to be investigated.
The value of long term sampling at more than one locality and within various habitats at each locality yielded several
answers and posed a number of questions. Should more samples be taken within the predominant habitat at a locality, for
example in the thickets and woodlands of a thicket locality and in the grasslands of a savanna locality, to obtain more
accurate results pertaining to an entire locality? What are the reasons for markedly more numerous questing ticks in a
treed savanna locality compared with a locality composed largely of thickets? What value can be added by simultaneously
sampling the major host species within a study site for ticks and determining host densities and daily and seasonal movements?
Finally, logistical challenges to what can be achieved in the field in a large nature reserve need to be considered, for
example (1) the dangers posed to field workers by large predators, African buffaloes, rhinoceroses and elephants, (2) the
number of drag samples that can be accomplished in a day to ensure reasonable homogeneity, as the total length of the
drags placed end to end covered a distance of 2250 m at each locality, and (3) the number of people, besides an armed
ranger, needed to collect, within a reasonable time frame, the thousands of ticks sometimes collected in a single drag.
We are indebted to the South African National Parks (SANParks) for placing their staff and facilities in the
KNP at our disposal. We gratefully acknowledge the assistance of Dr Leo Braack and Mr André Potgieter with
arranging the logistics for the collections. A number of nature conservation and veterinary students assisted
with many of the field collections. Our special thanks, however, are reserved for the SANParks staff at Skukuza,
who were our armed guards and assisted with the collection of the many thousands of ticks from the flannel strips.
The research was funded by the University of Pretoria (Faculty of Veterinary Science), SANParks, Bayer Animal
Health, the University of the Free State and the National Research Foundation.
Acocks, J.P.H., 1988, Veld types of South Africa with accompanying veld type map, 3rd edn.,
Department of Agriculture and Water Supply, Pretoria.
Apanaskevich, D.A., Horak, I.G. & Camicas, J-L, 2007, ‘Redescription of Haemaphysalis
(Rhipistoma) elliptica (Koch, 1844), an old taxon of the Haemaphysalis (Rhipistoma)
leachi group from East and southern Africa, and of Haemaphysalis (Rhipistoma) leachi
(Audouin, 1826) (Acarina, Ixodidae)’, Onderstepoort Journal of Veterinary Research 74, 181−208.
PMid:17933361
Apanaskevich, D.A. & Horak, I.G., 2008, ‘The genus Hyalomma. VI. Systematics of H.
(Euhyalomma) truncatum and the closely related species, H. (E.) albiparmatum
and H. (E.) nitidum (Acari, Ixodidae)’, Experimental and Applied Acarology 44,
115−136. doi:10.1007/s10493-008-9136-z,
PMid:18306046
Braack, L.E.O., Horak, I.G., Jordaan, L.C., Segerman, J. & Louw, J.P., 1996, ‘The comparative
host status of red veld rats (Aethomys chrysophilus) and bushveld gerbils (Tatera leucogaster)
for epifaunal arthropods in the southern Kruger National Park, South Africa’, Onderstepoort Journal
of Veterinary Research 63, 149−158.
PMid:8856764
Branch, W.R., 1998, Field guide to snakes and other reptiles of southern Africa, Struik, Cape Town.
Gallivan, G.J. & Surgeoner, G.A., 1995, ‘Ixodid ticks and other ectoparasites of wild ungulates in Swaziland:
regional, host and seasonal patterns’, South African Journal of Zoology 30, 169−177.
Gertenbach, W.P.D., 1983, ‘Landscapes of the Kruger National Park’, Koedoe 26, 9−121.
Horak, I.G., De Vos, V. & De Klerk, B.D., 1984, ‘Parasites of domestic and wild animals in South Africa. XVII.
Arthropod parasites of Burchell’s zebra, Equus burchelli, in the eastern Transvaal Lowveld’,
Onderstepoort Journal of Veterinary Research 51, 145−154.
PMid:6493729
Horak, I.G., Jacot Guillarmod, A., Moolman, L.C. & De Vos, V., 1987, ‘Parasites of domestic and wild animals
in South Africa. XXII. Ixodid ticks on domestic dogs and on wild carnivores’, Onderstepoort Journal of
Veterinary Research 54, 573−580.
PMid:3444612
Horak, I.G., Boomker, J., De Vos, V. & Potgieter, F.T., 1988, ‘Parasites of domestic and wild animals in
South Africa. XXIII. Helminth and arthropod parasites of warthogs, Phacochoerus aethiopicus, in the eastern
Transvaal Lowveld’, Onderstepoort Journal of Veterinary Research 55, 145−152.
PMid:3194114
Horak, I.G., Spickett, A.M., Braack, L.E.O. & Williams, E.J., 1991, ‘Parasites of domestic and wild animals in
South Africa. XXVII. Ticks on helmeted guineafowls in the eastern Cape Province and eastern Transvaal Lowveld’,
Onderstepoort Journal of Veterinary Research 58, 137−143.
PMid:1923375
Horak, I.G., Boomker, J., Spickett, A.M. & De Vos, V., 1992, ‘Parasites of domestic and wild animals in
South Africa. XXX. Ectoparasites of kudus in the eastern Transvaal Lowveld and the eastern Cape Province’,
Onderstepoort Journal of Veterinary Research 59, 259−273.
PMid:1297956
Horak, I.G., Spickett, A.M., Braack, L.E.O. & Penzhorn, B.L., 1993, ‘Parasites of domestic and wild animals in
South Africa. XXXII. Ixodid ticks on scrub hares in the Transvaal’, Onderstepoort Journal of Veterinary Research
60, 163−174.
PMid:7970571
Horak, I.G., Spickett, A.M., Braack, L.E.O., Penzhorn, B.L., Bagnall, R.J. & Uys, A.C., 1995, ‘Parasites of
domestic and wild animals in South Africa. XXXIII. Ixodid ticks on scrub hares in the north-eastern regions of Northern
and Eastern Transvaal and of KwaZulu-Natal’, Onderstepoort Journal of Veterinary Research 62, 123−131.
PMid:8600436
Horak, I.G., Braack, L.E.O., Fourie, L.J. & Walker, J.B., 2000a, ‘Parasites of domestic and wild animals in
South Africa. XXXVIII. Ixodid ticks collected from 23 wild carnivore species’, Onderstepoort Journal of
Veterinary Research 67, 239−250.
PMid:11206391
Horak, I.G., Spickett, A.M. & Braack, L.E.O., 2000b, ‘Fluctuations in the abundance of Boophilus decoloratus
and three Rhipicephalus species on vegetation during eleven consecutive years’, in M. Kazimírová, M. Labuda &
P.A. Nuttall (eds.), Proceedings of the 3rd International Conference on Ticks and Tick-borne Pathogens, into the 21st
century, Bratislava, Slovakia, 30 August–03 September, 1999, pp. 247–251.
Horak, I.G. & Cohen, M., 2001, ‘Hosts of the immature stages of the rhinoceros tick, Dermacentor rhinocerinus
(Acari, Ixodidae)’, Onderstepoort Journal of Veterinary Research 68, 75−77.
PMid:11403434
Horak, I.G., Emslie, F.R. & Spickett, A.M., 2001, ‘Parasites of domestic and wild animals in South Africa.
XL. Ticks on dogs belonging to people in rural communities and carnivore ticks on the vegetation’,
Onderstepoort Journal of Veterinary Research 68, 135−141.
PMid:11585091
Horak, I.G., Gallivan, G.J., Braack, L.E.O., Boomker, J. & De Vos, V., 2003, ‘Parasites of domestic and wild
animals in South Africa. XLI. Arthropod parasites of impalas (Aepyceros melampus) in the Kruger National
Park’, Onderstepoort Journal of Veterinary Research 70, 131−163.
PMid:12967174
Horak, I.G., Fourie, L.J. & Braack, L.E.O., 2005, ‘Small mammals as hosts of immature ticks’,
Onderstepoort Journal of Veterinary Research 72, 255−261.
PMid:16300195
Horak, I.G., Mckay, I.J., Heyne, H. & Spickett, A.M., 2006a, ‘Hosts, seasonality and geographic distribution
of the South African tortoise tick, Amblyomma marmoreum’, Onderstepoort Journal of Veterinary
Research 73, 13−25.
PMid:16715875
Horak, I.G., Gallivan, J., Spickett, A.M. & Potgieter, A.L.F., 2006b, ‘Effect of burning on the numbers of
questing ticks collected by dragging’, Onderstepoort Journal of Veterinary Research 73, 163−174.
PMid:17058438
Horak, I.G., Golezardy, H. & Uys, A.C., 2007, ‘Ticks associated with the three largest wild ruminant
species in southern Africa’, Onderstepoort Journal of Veterinary Research 74, 231−242.
PMid:17933365
Horak, I.G., Gallivan, G.J. & Spickett, A.M., 2011, ‘The dynamics of questing ticks collected for 164
consecutive months off the vegetation of two landscape zones in the Kruger National Park (1988–2002). I.
Total ticks, Amblyomma hebraeum and Rhipicephalus decoloratus’, Onderstepoort Journal of
Veterinary Research 78(1), Art. #32, 10 pages,
doi:10.4102/ojvr.v78i1.32
Keirans, J.E., 1993, ‘Dermacentor rhinocerinus (Denny, 1843) (Acari, Ixodida, Ixodidae), redescription of the
male, female and nymph and first description of the larva’, Onderstepoort Journal of Veterinary Research 60,
59−68.
PMid:8332318
Knapp, S.E., Krecek, R.C., Horak, I.G. & Penzhorn, B.L., 1997, ‘Helminths and arthropods of black
and white rhinoceroses in southern Africa’, Journal of Wildlife Diseases 33, 492−502.
PMid:9249695
Monadjem, A., 1997, ‘Habitat preferences and biomasses of small mammals in Swaziland’, African
Journal of Ecology 35, 64−72.
doi:10.1111/j.1365-2028.1997.042-89042.x
Neves, L., Afonso, S. & Horak, I.G., 2004, ‘Ixodid ticks on dogs in and around Maputo and elsewhere in Mozambique’,
Onderstepoort Journal of Veterinary Research 71, 279−283.
PMid:15732454
Norval, R.A.I. & Mason, C.A., 1981, ‘The ticks of Zimbabwe. II. The life cycle, distribution and hosts
of Rhipicephalus simus Koch, 1844’, Zimbabwe Veterinary Journal 12, 2−9.
Ogutu, J.O. & Owen-Smith, N., 2005, ‘Oscillations in large mammal populations: are they related to predation or
rainfall?’, African Journal of Ecology 43, 332−339.
doi:10.1111/j.1365-2028.2005.00587.x
Petney, T.N., Horak, I.G., Howell, D.J. & Meyer, S., 2004, ‘Striped mice, Rhabdomys pumilio, and other
murid rodents as hosts for immature ixodid ticks’, Onderstepoort Journal of Veterinary Research 71, 313−318.
PMid:15732458
Skinner, J.D. & Smithers, R.H.N., 1990, Mammals of the southern African subregion, University of Pretoria, Pretoria.
Spickett, A.M., Horak, I.G., Van Niekerk, A. & Braack, L.E.O., 1992, ‘The effect of veld-burning on the seasonal
abundance of free-living ixodid ticks as determined by drag-sampling’, Onderstepoort Journal of Veterinary Research
59, 285−292.
Spickett, A.M., Gallivan, G.J. & Horak, I.G., 2011, ‘The dynamics of questing ticks collected for 164
consecutive months off the vegetation of two landscape zones in the Kruger National Park (1988–2002). Part II.
Rhipicephalus appendiculatus and Rhipicephalus zambeziensis’, Onderstepoort Journal of Veterinary
Research 78(1): Art. #233, 9 pages.
doi:10.4102/ojvr.v78i1.233,
PMid:1297958
Theiler, G. & Salisbury, L.E., 1959, ‘Ticks in the South African Zoological Survey Collection – Part IX
– “The Amblyomma marmoreum group”’, Onderstepoort Journal of Veterinary Research 28, 47−124.
Uys, A.C. & Horak, I.G., 2005, ‘Ticks on crested francolins and vegetation in Limpopo Province,
South Africa’, Onderstepoort Journal of Veterinary Research 72, 349−343.
Walker, J.B., Keirans, J.E. & Horak, I.G., 2000, The genus Rhipicephalus (Acari, Ixodidae), a guide
to the brown ticks of the world, Cambridge University Press, Cambridge.
|
|