|
Article Information
|
Authors:
Dirk G. Booyse1
Burk A. Dehority2
Affiliations:
1Department of Anatomy and Physiology, University of Pretoria, South Africa
2Department of Animal Sciences, Ohio State University, USA
Correspondence to:
Dirk Booyse
Email:
dbooyse@op.up.ac.za
Postal address:
Private Bag X04, Onderstepoort 0110,
South Africa
Dates:
Received: 02 Mar. 2011
Accepted: 23 May 2011
Published: 12 Oct. 2011
How to cite this article:
Booyse, D.G. & Dehority, B.A., 2011, ‘Protozoa and digestive tract parameters of the impala’, Onderstepoort Journal of Veterinary
Research 78(1), Art. #327, 5 pages.
doi:10.4102/ojvr.v78i1.327
Copyright Notice:
© 2011. The Authors. Licensee: AOSIS OpenJournals. This work
is licensed under the Creative Commons Attribution License.
ISSN:
0030-2495 (print)
ISSN:
2219-0635 (online)
|
|
|
|
Protozoa and digestive tract parameters of the impala
|
|
In This Original Research...
|
Open Access
|
• Abstract
• Introduction
• Materials and methods
• Animals
• Feed
• Measurements
• Protozoa
• Results and discussion
• Protozoa
• Physiological parameters
• Conclusion
• Acknowledgements
• Authors’ contributions
• References
|
|
Intestinal contents were collected from eight impala at three different localities during the winter hunting season (2005–2009), as well as from another
24 animals from a one-year trial at a game farm called Ditholo (2003–2004). Gas production, protozoa counts and several other physiological parameters were
measured from both rumen and caecum or colon contents. Only higher ophryoscolecid and Isotrichidae species of protozoa were counted and identified. Ostracodinium
gracile was present in all 32 impala. Eudiplodinium maggii was present in 31 animals and Eudiplodinium impalae and Epidinium (either
ecaudatum or caudatum) in 30 animals. Dasytricha ruminantium was present in only 11 of the impala. Concentrations of protozoa were
correlated with the season of sample collection and highly correlated with the animals living on the game farm. Gas production (mL/g of wet rumen ingesta) was
weakly correlated with protozoa concentration but not with the season of collection.
A total of 13 protozoan species have previously been reported from the rumen of the impala. Dogiel (1925) listed six protozoan species from impala in Kenya and
later, in 1932, he observed five species in Uganda. Four species were the same in both locations and the fifth belonged to the same genus but was identified as a
different species (Table 1). In South Africa, Van der Wath and Myburgh (1941) reported the presence of three species in impala rumens and later Kleynhans (1977)
recorded the presence of 11 species. Four of the species observed by Kleynhans, Isotricha prostoma, Entodinium parvum, Eudiplodinium maggii
and Dasytricha ruminantium, were new host records for impala (see Table 1). Dehority and Odenyo (2003) identified protozoa in impala from Kenya only to
the generic level and did not find any genera in which species had not previously been observed.
Apart from the counting and identification of protozoa, several other parameters of the digestive tract of the impala (rumen, small intestine, caecum and colon)
were also investigated and are reported here for the first time. These include pH and temperature of contents, length of organ, contents weight per organ, gas
production, dry matter and density.
Animals
One female animal was collected from a farm called Karoobult, 100 km north-west of Pretoria (24°43’49.45”S, 27°33’22.88”E),
in July 2009, five animals (three male and two female) were from the Loskop Dam Nature Reserve in Mpumalanga province, 200 km east of Pretoria
(25°25’51.91”S, 29°19’39.80”E), and two male animals were from the Rietvlei Nature Reserve, 50 km south of Pretoria
(25°52’41.17”S, 28°16’17.83”E). In addition, 24 animals (all male) were from a one-year trial from the Ditholo game farm,
60 km north of Pretoria (25°19’43.95”S, 28°19’04.34”E).
Feed
Impala are mixed feeders and therefore graze as well as browse. Feed available to the impala consists of vegetation types called bushvelds. A bushveld can be
described as a heavily grassed plain, dotted by dense clusters of trees and tall shrubs. The grasses found here are generally tall and turn yellow or brown in
winter, which is the dry season throughout most of South Africa. Sour bushveld consists of grazing fields, mostly comprising grasses like Eragrostis curvula,
Eragrostis chloromelas, Hyparrhenia hirta and Cymbopogon excavatus. These grasses are fast-growing plants in the summer, but die rapidly in
winter, turning hard and tasteless. Sour grass species can, however, recover quite rapidly from overgrazing. Sweet bushveld generally consists of
grasses that grow slowly in summer but tend to stay highly nutritious and palatable during the dry season (winter). Sweet grass species include Digitaria
eriantha, Panicum maximum and Urochloa mosambicensis. Sweet grasses recover very slowly from heavy impacts like overgrazing. Mixed bushveld
is a field composed of both sour and sweet grasses. The veld type in the Rietvlei and Ditholo areas is described as a sourish mixed bushveld, whilst Karoobult
represents sour bushveld and the vegetation from the Loskop area is described as a mixed bushveld. In the spring and summer, impala both feed on grass
(Eragrostis) and browse on trees like Acacia karoo and Acacia caffra. However, in the winter they are forced to ‘low graze’
on grass, as little or no browse is available.
Measurements
After shooting, the animals were weighed, the digestive tract removed and the different anatomical sections (rumen, small intestines, caecum and colon) were ligated
before separation. Each section was weighed and the temperature, pH and length of the organs were measured and noted. Weights were measured with an electronic scale
capable of measuring up to two decimal places and pH was measured with a portable, battery-powered pH meter (Eutech, model EC-PH-10/01N, Singapore). Organ content
weights were estimated by weighing the organ full and empty.
Samples for fermentation studies (200 mL) were placed in fermentation vessels and gas production was measured every minute by means of a glass syringe (fitted
with a 20-gauge stainless steel needle), inserted through the rubber stopper. Measurements were taken over a period of 45 min. The exact weight of the samples
was determined after complete fermentation.
Protozoa
A large sample of ingesta was taken from the rumen and the fluid squeezed out into a 40-mL plastic specimen bottle. For the caecum and colon samples, small aliquots
were taken at random from the contents and poured into the 40-mL container. These samples were then transferred to larger marked containers and 100 mL 70%
methanol was added to each. Back at the laboratory, the protozoa samples were washed through sieves as described previously (Booyse, Boomker & Dehority 2010).
In essence, the entire sample was washed through a set of sieves with an inner and outer chamber. The sample was poured into the inner sieve (pore size 110 μm)
and washed with water. Particulate matter and protozoa passed through to the outer sieve, which had a pore size of 37 μm. The outer sieve was fitted with a
draining tap to allow the contents to be drained into a bottle. The washed sample was then allowed to stand for 30 min and the volume adjusted back to 40 mL
by decanting the excess water. Two drops of Brilliant Green stain were added to each of the 40-mL samples, which were then allowed to stand for 24 h (Dehority
1984). Three aliquots (0.1 mL each) from each sample were placed onto separate microscope slides and fitted with a cover slip. Protozoa on each slide were counted
using a standard light microscope fitted with a Panasonic digital camera (model GP220). Concentrations were determined by multiplying the mean of all three counts by
10, thus giving a count per millilitre.
Protozoa
Initial examination of the protozoa samples revealed – unexpectedly – a complete absence of Entodinium species. Subsequent investigation revealed
that these species were washed out through the 37-µm pores. The samples were washed primarily to remove most of the larger plant debris, which interferes with
microscopical observation. Because all the samples had been washed, we were able to enumerate only Isotrichidae and the subfamilies Diplodiniinae and Ophryoscolecinae
in the family Ophryoscolecidae (larger ophryoscolecid protozoa). However, we were able to shoot an additional impala, collect rumen contents, stain and count protozoa
in a 0.1-mL sample directly, without washing through sieves. Our examination revealed the presence of numerous Entodinium species in this last animal, which
were identified and included with our previous data in Table 1.
|
TABLE 1: Rumen protozoa observed from impala (Aepyceros melampus).
|
Four earlier reports on protozoa in the impala are also included in Table 1 for comparison. To date, 13 species of protozoa have been observed in the impala rumen.
In South Africa, 11 of these were reported by Kleynhans in 1977, nine in the present study and three by Van der Wath and Myburgh in 1941. Dogiel reported a total of
five species from impala in Kenya (1925) and Uganda (1932).
Table 2 reflects the occurrence and concentration of D. ruminantium and the larger ophryoscolecids (five species) as grouped by the collection site.
Ostracodinium gracile was present in all 32 animals, E. maggii was present in 31 animals, whilst Eudiplodinium impalae and Epidinium
caudatum were found in 30. In contrast, Epidinium ecaudatum was present in only 16 animals and Dasytricha species were found in nine. Thus, the
majority of the larger ophryoscolecids were represented by four species, namely O. gracile, E. maggii, E. impalae and Ep. caudatum. The
total concentration of all six species averaged about 1.6 x 104 per mL in the 24 animals from Ditholo, 0.32 x 104 per mL
in the five from Loskop, 1.38 x 104 per mL in the two impala from Rietvlei and 0.28 x 104 per mL in the single animal from Karoobult.
|
TABLE 2: Occurrence and concentration of Dasytricha ruminantium and larger ophryoscolecid protozoa in rumen contents of the impala.
|
The caecum and colon contents were mixed, washed as described above, and examined microscopically for protozoa. The results in Table 3 show that only very low
concentrations were present in the ingesta from these organs. Presumably, the protozoa would be digested as they move through the abomasum and small intestine;
however, some cells are obviously able to pass through unharmed. It is possible that they are embedded in larger food particles and are not exposed to the acidic
and enzymatic conditions in those organs. These concentrations are considerably higher than what one author (B.A.D.) has previously observed in domestic ruminants
(unpublished).
|
TABLE 3: Occurrence and concentration of Dasytricha ruminantium and larger ophryoscolecid protozoa in the caecum or colon contents of impala.
|
A possible relationship between the time (i.e. the month when the animal was harvested) and the concentration of the larger protozoa was investigated, taking all
32 animals into consideration. A correlation coefficient (r) of 0.65 was obtained (r2 = 0.42). However, when only the 24 animals from a
single location (Ditholo) were used, the correlation coefficient was 0.865 (r2 = 0.75). Figure 1 shows the regression of protozoa concentration for
all 32 animals, according to time of harvesting. The first sample was obtained in December and labeled as 0, followed by January as 1, February as 2, etc. This was
based on the assumption that the start of the summer growing season would begin in December, when pasture and browse were low, and that feed would become increasingly
available during the following months.
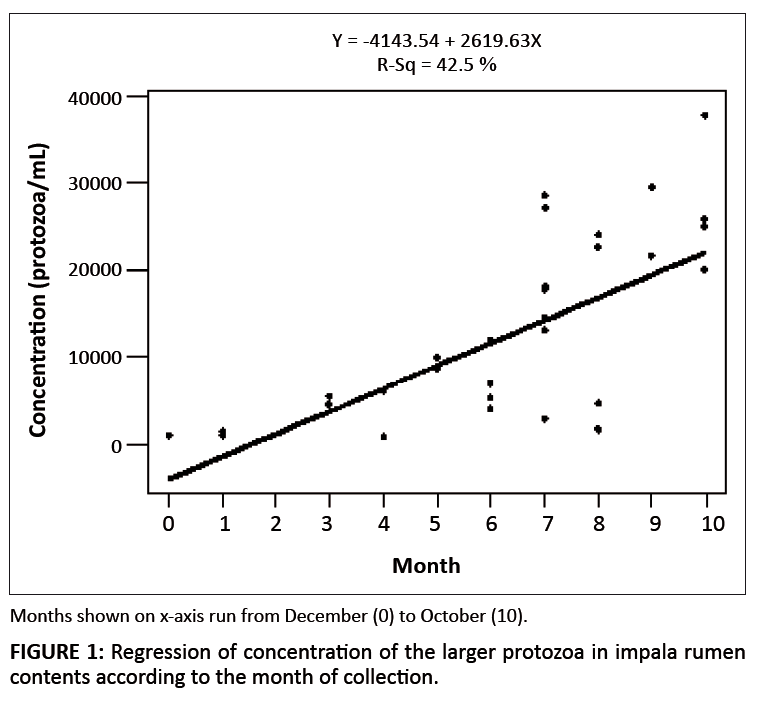 |
FIGURE 1: Regression of concentration of the larger protozoa in impala rumen
contents according to the month of collection.
|
|
It is also of interest that only two genera of the subfamily Diplodiniinae, Eudiplodinium and Ostracodinium, have been found in the impala. Species of
Diplodinium, Metadinium, Enoploplastron, Elytroplastron and Polyplastron occur in many of the other wild ruminants in Africa (Dehority &
Odenyo 2003; Dogiel 1925, 1932; Kleynhans & Van Hoven 1975; Van Hoven 1975, 1978, 1983).
Physiological parameters
Table 4 presents information on the collection site, age, sex, organ pH, weight of the organ contents, percentage dry matter, density and gas production from
organ contents, incubated in vitro (gas production was not measured with small intestine ingesta). Except for the animal from Karoobult and the two from
Rietvlei, rumen pH values averaged below 6.0. This value would be expected in grazing ruminants, as demonstrated in the study by Dehority and Tirabasso (2001),
where sheep were fed 1, 6, or 24 times per day. Rumen dry matter values fell within the normal reported range of 10% – 13% (Dehority 2003).
|
TABLE 4: Physiological parameters of the impala digestive tract and gas production by rumen, caecum and colon contents.
|
The aim of measuring gas production was to evaluate whether this parameter was associated with protozoal concentrations. When gas production was correlated with
numbers of larger protozoa, the correlation coefficient was 0.31, indicating little or no association. This is not entirely unexpected, because a number of factors
would be involved. Probably the most critical would be the amount of available substrate in the rumen contents, regardless of the concentration of protozoa and
bacteria. Gas production from rumen, caecum and colon contents was greater in the animals on the game farm, suggesting that more substrate was available in the
ingesta from these animals than from those living in the bushveld. In future studies it would be desirable to measure fermentation capacity as described by El-Shazley
and Hungate (1965), where additional substrate is added.
Temperature of organ contents ranged from 30 ºC to 41 ºC for the rumen, between 11 ºC and 28 ºC for the small intestine, between
17 ºC and 31 ºC for the caecum and between 13 ºC and 30 ºC for the colon. Length of the organs ranged from 281 mm to
570 mm for the rumen, 6218 mm to 13 852 mm for the small intestine, 162 mm to 652 mm for the caecum, and 190 mm to 1411 mm
for the colon (data not shown).
The number of genera and species of protozoa occurring in the impala is limited compared to other African wild ruminants and domesticated cattle and sheep
(Booyse & Dehority in press; Dehority & Odenyo 2003; Dogiel 1932; Kleynhans & Van Hoven 1976; Van Hoven 1975, 1978, 1983). Only four species of
Entodinium, two genera of Diplodiniinae and two species of Epidinium have been observed.
Authors’ contributions
D.G.B. collected the samples, was responsible for all the measurements and conducted the microscopic studies. B.A.D. assisted with identification of the species,
compilation of the data and writing the manuscript.
Booyse, D.G. & Dehority, B.A., in press, ‘Rumen protozoa in South African sheep with a summary of the worldwide distribution of sheep
protozoa’, Onderstepoort Journal of Veterinary Research.
PMid:2628563
Booyse, D.G., Boomker, E.A. & Dehority, B.A., 2010, ‘Protozoa in the digestive tract of wild herbivores in South Africa. I: Warthogs (Phacochoerus
aethiopicus)’, Zootaxa 2492, 63–68.
Dehority, B.A., 1984, ‘Evaluation of sub-sampling and fixation procedures used for counting rumen protozoa’, Applied and Environmental Microbiology
48, 182–185.
PMid:6476828,
PMid:240360
Dehority, B.A., 2003, Rumen Microbiology, Nottingham University Press, Nottingham, UK.
Dehority, B.A. & Odenyo, A.A., 2003, ‘Influence of diet on the rumen protozoal fauna of indigenous African wild ruminants’, Journal of
Eukaryotic Microbiology 50, 220–223.
Dehority, B.A. & Tirabasso, P.A., 2001, ‘Effect of feeding frequency on bacterial and fungal concentrations, pH and other parameters in the rumen’,
Journal of Animal Science 79, 2908–2912.
PMid:11768121
Dogiel, V., 1925, ‘Nouveaux infusoires de la famille des Ophryoscolécidés parasites d’antilopes Africaines’ [New infusoria of the
family of ophryoscolecid parasites in African antelope], Annales de Parasitologie 2, 116–142.
Dogiel, V., 1932, ‘Beschreibung einiger neuer Vertreter der Familie Ophryoscolecidae aus afrikanischen Antilopen nebst Revision der Infusorienfauna afrikanischer
Wiederkäuer‘ [Description of some new representatives of the family Ophryoscolecidae from African antelope together with a revision of the infusorial fauna
of African ruminants], Archiv für Protistenkunde 77, 92–107.
El-Shazley, K. & Hungate, R.E., 1965, ‘Fermentation capacity as a measure of net growth of rumen organisms’, Applied Microbiology 13,
62–69.
PMid:14269247,
PMid:1058191
Kleynhans, C.J., 1977, ‘The rumen protozoa of the impala (Aepyceros melampus) Lichtenstein, and the kudu (Tragelaphus strepsiceros) Pallas’,
PhD thesis, Department of Zoology, University of Pretoria.
Kleynhans, C.J. & Van Hoven, W., 1976, ‘Rumen protozoa of the giraffe with a description of two new species’, East African Wildlife
Journal 14, 203–214.
Van der Wath, J.G. & Myburgh, S.J., 1941, ‘Studies on the alimentary tract of merino sheep in South Africa. VI. The role of infusoria in ruminal
digestion with some remarks on ruminant bacteria’, Onderstepoort Journal of Veterinary Science and Animal Industry 17, 61–85.
Van Hoven, W., 1975, ‘Rumen ciliates of the Tsessebe (Damaliscus lunatus lunatus) in South Africa’, Journal of Protozoology 22, 457–462.
PMid:811788
Van Hoven, W., 1978, ‘Development and seasonal changes in the rumen protozoan population in young blesbok (Damaliscus dorcas Phillipsi Harper 1939)’,
South African Journal of Wildlife Research 8, 127–130.
Van Hoven, W., 1983, ‘Rumen ciliates with descriptions of two new species from three African reedbuck species’, Journal of Protozoology 30,
688–691.
|
|
